Hydrogen Bonding
Hydrogen Bonding: Overview
This topic consists of various concepts like Intra-molecular Hydrogen Bond,Effect of Intermolecular Hydrogen Bonding on Boiling Point,Inter-molecular Hydrogen Bond, etc.
Important Questions on Hydrogen Bonding
In an ice crystal, each water molecule is hydrogen bonded to _____ neighbouring molecules.
For the correct order of increasing extent of hydrogen bonding is:
Which of the following statement is not true on the basis of hydrogen bonding?
Which compound form polymer due to hydrogen bond?
The molecules that can form intramolecular hydrogen bonding are
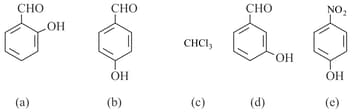
How many of the given compounds show hydrogen bonding?
Phenol, , cyclohexane
Maximum number of hydrogen bonds per molecule of water is
Which gas is most soluble in water?
The intramolecular hydrogen bonding in compound leads to:
-nitrophenol and -nitrophenol are separated by
Among the common mineral acids, is less volatile due to
Identify the correct sequence with respect to the strength of hydrogen bonding among the following.
The dominant intermolecular force that must be overcome to convert liquid methanol to its vapour is _______
The correct order of boiling point in the following compounds is
The increasing order of the boiling points for the following compounds is:
The number of hydrogen bonds formed by a water molecule at normal conditions is
Which of the molecules does not show hydrogen bonding?
Which of following forms intramolecular hydrogen bond after removal of most acidic
Check the following molecules and identify in which of the following substances will hydrogen bond be the strongest?
Which of the following molecule doesn't show hydrogen bonding?
